Neuropeptide Y5 receptor
Food intake in free-feeding and energy-deprived lean rats is
mediated by the neuropeptide Y5 receptor
Authors
L Criscione, P Rigollier, C Batzl-Hartmann, H Rüeger, A Stricker-Krongrad, P Wyss, L Brunner, S Whitebread, Y Yamaguchi, C Gerald, R O Heurich, M W Walker, M Chiesi, W Schilling, K G Hofbauer, N Levens
Food Intake in Free-feeding and Energy-deprived Lean Rats Is Mediated by the Neuropeptide Y5 Receptor
Leoluca Criscione,* Pascal Rigollier,* Christine Batzl-Hartmann,* Heinrich Rueger,* Alain Stricker-Krongrad,* Philipp Wyss,* Liliane Brunner,* Steven Whitebread,* Yasuchika Yamaguchi,* Christoph Gerald,* Rainer O. Heurich,* Mary W. Walker,* Michele Chiesi,* Walter Schilling,* Karl G. Hofbauer,* and Nigel Levens*
*Metabolic and Cardiovascular Diseases Research, Novartis Pharma AG, CH-4002 Basel, Switzerland; and ^Synaptic Pharmaceutical Corporation, Paramus, New Jersey 07652
Version 1 (December 15, 1998):
Abstract
The new neuropeptide Y (NPY) Y5 receptor antagonist CGP 71683A displayed high affinity for the cloned rat NPY Y5 subtype, but > 1,000-fold lower affinity for the cloned rat NPY Y1, Y2, and Y4 subtypes. In LMTK cells transfected with the human NPY Y5 receptor, CGP 71683A was with- out intrinsic activity and antagonized NPY-induced Ca2' transients. CGP 71683A was given intraperitoneally (dose range 1-100 mg/kg) to a series of animal models of high hy- pothalamic NPY levels. In lean satiated rats CGP 71683A significantly antagonized the increase in food intake in- duced by intracerebroventricular injection of NPY. In 24-h fasted and streptozotocin diabetic rats CGP 71683A dose- dependently inhibited food intake. During the dark phase, CGP 71683A dose-dependently inhibited food intake in free-feeding lean rats without affecting the normal pattern of food intake or inducing taste aversion. In free-feeding lean rats, intraperitoneal administration of CGP 71683A for 28 d inhibited food intake dose-dependently with a maximum reduction observed on days 3 and 4. Despite the re- turn of food intake to control levels, body weight and the pe- ripheral fat mass remained significantly reduced. The data demonstrate that the NPY Y5 receptor subtype plays a role in NPY-induced food intake, but also suggest that, with chronic blockade, counterregulatory mechanisms are in- duced to restore appetite. (J. Clin. Invest. 1998. 102:2136- 2145.) Key words: obesity ♦ energy metabolism ♦ weight re- duction ♦ neuropeptide Y ♦ insulin
Introduction
Within the hypothalamus, neuropeptide Y (NPY)1 is inti- mately involved in the regulation of several aspects of neu- roendocrine function and behavior, in particular food intake
Address correspondence to Leoluca Criscione, Novartis Pharma AG, K-125.801, CH-4002 Basel, Switzerland. Phone: 41-61-6965137; FAX: 41-61-6965561; E-mail: Leoluca.criscione@pharma.novartis.com
Received for publication 4 June 1998 and accepted in revised form 28 October 1998.
1. Abbreviations used in this paper: ARC, arcuate nucleus; DMH, dorsomedial hypothalamus; hPYY, human peptide YY; NPY, neu- ropeptide Y; PVN, paraventricular nucleus.
J. Clin. Invest.
© The American Society for Clinical Investigation, Inc. 0021-9738/98/12/2136/10 $2.00
Volume 102, Number 12, December 1998, 2136-2145 https://www.jci.org
(1). For example, acute injection of NPY into the cerebral ven- tricles or directly into the hypothalamus leads to substantially increased food intake in satiated rats (2, 3). In free-feeding rats, both hypothalamic NPY and NPY mRNA are increased on transition from the light to the dark phase of the day/night cycle whereas, in both fasted and streptozotocin diabetic rats, two models of energy deficit, NPY levels and NPY mRNA are also increased in this brain region (4-11).
A temporal relationship between increased hypothalamic NPY levels and food intake has been established (12, 13). Fur- thermore, inhibition of NPY synthesis or blockade of the ac- tions of NPY with antibodies or NPY antisense oligonucleotides has been shown to reduce food intake in both free-feeding and food-deprived rats (14-16). These studies, along with the changes in NPY levels in the hypothalamus described above, all suggest that NPY plays a physiologically important role in the control of food intake in both normal and energy- deprived rats.
The actions of NPY are believed to be mediated by several receptor subtypes named NPY Y1-Y6 (17). Recent studies cor- relating the NPY Y5 binding affinity of a range of NPY peptide agonists with their ability to induce food intake suggest that this receptor subtype is intimately involved in NPY-induced feeding (18, 19). This conclusion is supported by the substan- tial reduction in food intake observed in fasted rats after in- tracerebroventricular injection of antisense oligonucleotides directed against the NPY Y5 receptor (20, 21). However, clouding the issue are observations with selective peptide ago- nists and nonpeptide antagonists pointing to the involvement of the NPY Y1 receptor or other, currently unidentified receptor subtypes in the control of NPY-induced food intake (2227). Therefore, despite extensive investigation, these conflict- ing observations have not enabled a definition of the receptor subtype(s) mediating the increase in food intake produced by NPY to be clearly established.
In view of the above controversy, the purpose of the fol- lowing experiments was to investigate the hypothesis that the NPY Y5 receptor subtype plays a major role in mediating food intake induced by NPY. This hypothesis was examined by studying NPY-induced feeding behavior in response to block- ade of NPY Y5 receptors with a new highly selective nonpep- tide antagonist, CGP 71683A.
Methods
Animals. These studies were conducted with lean male Sprague- Dawley rats (250-380 g, Tif: RA25; Novartis Pharma AG, Basel, Switzerland). The animals were maintained in rooms with a 12-h light/dark cycle (light 8 a.m. to 8 p.m.) at 20-24°C and controlled hu- midity. All animals were given tap water to drink and were main- tained on a normal pellet diet (NAFAG, Gossau, Switzerland) for at least 1 wk before use in the following experiments.
Determination of the binding affinity of CGP 71683A to rat NPY receptor subtypes. HEK-293 cells stably expressing the rat NPY Y5 receptor subtype or LMTK cells stably expressing the rat NPY Y1, Y2, or Y4 receptor subtypes (18) were homogenized in ice-cold hypotonic buffer (Tris-HCl 20 mM, EDTA 5 mM; pH 7.7 at 4°C). The homoge- nate was centrifuged at 32,000 g for 20 min at 4°C and the resultant pellet was resuspended in the same buffer and recentrifuged. The fi- nal pellet was suspended at room temperature in Hepes buffer (Hepes 20 mM, NaCl 10 mM, CaCl2 1.26 mM, MgSO4 0.81 mM, KH2PO4 0.22 mM, pH 7.4). Shortly before use, 0.1% BSA was added. The protein content of the pellet was determined by the method of Bradford using BSA as standard (28).
Competition binding studies were performed in Millipore Multi- screen FB filter plates at room temperature for 2 h. The filters in each well were pretreated with 2% polyethyleneimine for 30 min and rinsed once with 300 ml Hepes buffer before use. The following were pipetted into each well: 60 ml Hepes buffer, 20 ml 125I-[Pro34]human peptide YY (hPYY) (for the NPY Y1, Y4, and Y5 binding studies or 125I-hPYY for the NPY Y2 binding studies; 600 pM; Anawa, Wangen, Switzerland) in Hepes buffer; 20 ml CGP 71683A in Hepes buffer plus 10% DMSO (or Hepes buffer plus 10% DMSO for the controls); 100 ml crude membrane suspension (z 10 mg protein). The incubations were terminated by rapid filtration and washing four times with 300 ml PBS. The filters were removed from the wells, placed into plastic tubes, and counted in a g-counter (Wallac, Turku, Finland). Nonspe- cific binding was defined as the binding remaining in the presence of
- mM unlabeled [Pro34]hPYY (for the NPY Y1, Y4, and Y5 binding studies) or hPYY (for the NPY Y2 binding studies).
Measurement of intracellular Ca21 transients in LMTK cells stably expressing the human NPY Y5 receptor. T175 NUNC plates of conflu- ent LMTK cells were harvested in EDTA/PBS medium, washed in PBS, and then loaded for 90 min at room temperature with 10 mM FLUO-3 AM in 120 mM NaCl, 1 mM MgCl2, 5.4 mM KCl, 0.33 mM NaH2PO4, 11 mM glucose, 5 mM taurine, 2 mM Na-pyruvate, 1.5 mM L-glutamine, 10 U/liter insulin, 0.1% BSA, 1 mM CaCl2, and 10 mM Hepes, pH 7.4, supplemented with pluronic acid (10 ml/plate). After centrifugation, the cells were resuspended at a concentration of 5-6 million cells/ml in buffer (see above) supplemented with 250 mM sulfinpyrazone.
Intracellular Ca21 transients were measured at room temperature in a microtiter plate using a Cytofluor 2350 fluorometer (Millipore Corp., Bedford, MA) with wavelength settings at 485 nm for excita- tion and 530 nm for emission. The ability of NPY or other com- pounds to induce Ca21 transients was determined in triplicate by add- ing 30 ml of the agent (dissolved in 2 ml DMSO and diluted with 28 ml of buffer) to 170 ml of cells. Antagonistic activity was determined in triplicate by incubating 170 ml cells at room temperature in the pres- ence of various quantities of compounds dissolved in 2 ml DMSO (or
- ml DMSO for the controls) for 5 min. Thereafter, 30 ml of human NPY in buffer was added to reach a final concentration of 100 nM and intracellular Ca21 changes were monitored. After 10 min, when the intracellular Ca21 concentration had returned to basal levels, 30 ml of the thrombin receptor agonist peptide Ser-(p-Fluoro)Phe-Leu- (b-cyclohexyl)Ala-ArgNH2 (TRAP) was added to reach a final concentration of 10 mM. NPY selective antagonists should show no effect on TRAP-induced Ca21 transients. Ca21 transient curves were ob- tained after subtraction of the control curves. The compound concen- tration giving 50% inhibition of the maximum of the Ca21 transients induced by NPY was then calculated.
Intracerebroventricular cannulation. Under pentobarbital anes- thesia (50 mg/kg i.p.; Veterinaria AG, Zurich, Switzerland) each rat was stereotaxically implanted with an 8-mm-long guide cannula (0.6 mm O.D., 0.35 mm I.D.) aimed at the right lateral ventricle. With the incisor bar set 2 mm below the interaural line, the stereotaxic coordi- nates were —0.8 mm anterior and 11.3 mm lateral to the bregma. The rats were allowed 7 d of recovery, during which they were handled daily to minimize nonspecific stress. On the first day of study, all ani- mals received an intracerebroventricular injection of human angio- tensin II (150 ng/rat, Hypertensin; Novartis Pharma AG). Only those animals that responded with a sustained drinking response within 2 min were used in the following experiments, which were performed 1 wk after the injection of angiotensin II.
Measurement of food intake. In the majority of experiments de- scribed in these studies, food intake was assessed after placing the rats into individual plastic metabolism cages (Techniplast; Indulab, Garns, Switzerland). During these studies the rats had free access to powdered regular food and tap water. After 6 d of habituation to their new environment, food intake over both the light and dark phases was measured, to establish baseline food intake for each ani- mal. Thereafter, the rats were randomly assigned to either vehicle- or drug-treatment groups. At 8 a.m. on the day of study, the animals were injected intraperitoneally with either vehicle (10% DMSO, 2 ml/kg) or differing doses of CGP 71683A in vehicle. Food intake was then measured for time periods up to 24 h (acute experiments, single intraperitoneal injection) or over the complete light and dark phases for times up to 28 d (chronic experiments, single daily intraperitoneal injections). Because of their design, food spillage from the metabo- lism cages was minimal and was not corrected for.
For practical reasons, experiments involving the intracerebroven- tricular injection of NPY were performed individually in wire-topped plastic cages. Before the test procedure, a preweighed quantity of food (five pellets weighing z 45 g) was placed on the roof grids. At 8 a.m. on the day of study, each rat was injected intracerebroventricu- larly with either 300 pmol NPY or an equivalent volume of the artifi- cial cerebrospinal fluid vehicle (10 ml) and then returned to their cages. After injection, the food on the cages was weighed at 1-h inter- vals for the remainder of the experimental period. Food spillage was monitored and, if necessary, food intake was corrected for this.
Measurement of water intake. Rats were maintained in plastic metab- olism cages as described above. After 6 d of habituation to their new environment, both water and food intake over the light and dark phases were measured to establish baseline values for each animal. Thereafter, the rats were randomly assigned to either vehicle or drug treatment groups and deprived of both food and water for the 24-h period before study. At 8 a.m. on the day of study, the animals were injected intraperitoneally with either vehicle (10% DMSO, 2 ml/kg) or differing doses of CGP 71683A in vehicle (1, 10, and 100 mg/kg). Water intake was then measured for time periods up to 24 h.
Measurement of the pattern of food intake. Each cage included a complete automatic feeding system connected to a computer. After 2 wk of habituation, all rats were injected intraperitoneally either with vehicle (10% DMSO, 2 ml/kg) or with 10 mg/kg CGP 71683A dissolved in vehicle. All injections were performed at the beginning of the light period (8 a.m.). After injection, the animals were put back into the feeding system and their feeding behavior was analyzed until the end of the dark period, e.g., during the 24 h that followed injec- tion. Before the first injection, an intraanimal calculation was per- formed three times during a minimum of 24 h to ensure that the ani- mals were well adapted to the system.
A meal was defined as ingestion of a minimum of 0.2 g of the diet followed by at least 10 min during which no feeding occurred. The following dependent variables and derived measures were calculated as the mean of each variable for each animal: meal number, total amount of food eaten (g), meal intervals (min), meal size (g), meal duration (min), eating rate (g/min), and time to first meal (min). Re- cording of the impulses generated was performed by a microcom- puter through an in/out interface (Ciba Pharma Electronic PH2, DA32; Buxco Electronics, Sharon, CT). Data from the feeding sys- tem were analyzed with specific software developed in Excel™ (ver- sion 5.1) (29).
Conditioned taste aversion. Conditioned taste aversion in response to CGP 71683A was adapted from previously published methods (30, 31). The rats in this study were maintained individually in plastic cages and, after a 7-d habituation period, were deprived of water for 14 h (12 p.m. to 2 p.m.) on each of five consecutive days (training ses- sions). During this period, water was returned to the animals for the remaining 10 h after water deprivation. Water intake within the first 50 min (day 1), 30 min (days 2 and 3), and 20 min of water repletion (days 4 and 5) was measured by weighing the drinking bottles. 2 d af- ter the last water deprivation, the animals were water deprived for 14 h but offered a solution of sodium saccharine (0.2% wt/vol) instead of water (test day 1). The intake of the saccharine solution was mea- sured for 20 min and, 15 min later, the animals were injected intraper- itoneally with either a solution of lithium chloride (10, 100, and 1,000 mmol/kg) or CGP 71683A (1, 10, and 100 mg/kg). Control animals re- ceived an equal volume of the 10% DMSO vehicle solution (2 ml/kg, i.p.). 2 d later, after 14 h of water deprivation, the rats were offered the saccharine solution again and the quantity consumed in 20 min was measured (test day 2). Conditioned taste aversion was determined as saccharine intake (ml/20 min) in the lithium chloride-treated or CGP 71683A-treated rats compared with Controls on test day 2.
Streptozotocin diabetic rats. Rats were injected intraperitoneally with streptozotocin (65-70 mg/kg) or the 1% ascorbic acid vehicle (2 ml/kg). Because of the large number of rats studied (60), the experi- ments had to be conducted in two equal groups. 2 wk after injection, the rats were placed into metabolism cages and randomly assigned to one of four treatment groups in a balanced design. After 3 d of habit- uation, each group of rats was injected intraperitoneally at the begin- ning of the light phase with either CGP 71683A (1, 10, and 100 mg/kg) or the 10% DMSO vehicle (2 ml/kg). Cumulative food intake was measured at various time intervals over the next 24-h period. At the end of the experiment, each rat was decapitated and the trunk blood was collected into ice-cold heparinized tubes. The blood was centri- fuged at 4°C and the plasma was harvested and stored frozen at —80°C before the measurement of plasma glucose and insulin con- centrations. In the first group of rats those animals exhibiting plasma insulin levels > 2 ng/ml, an indication that diabetes had not been in- duced, were excluded from the subsequent data analysis. It was noted in the first group of rats that those animals having average food in- takes in the 48 h before study of > 28 g/24 h were normally those with plasma insulin > 2 ng/ml. Therefore, in the second experimental group, four animals that had food intakes > 28 g/24 h before the study were excluded from the study prior to the injection of CGP 71683A. None of the remaining animals in the second group had plasma insulin > 2 ng/ml.
Plasma measurements. Glucose was measured with an enzymatic method using a commercially available reagent (Beckman, Galway, Ireland). Plasma insulin was determined by radioimmunoassay using a commercially available kit (Sorin Biomedica, Saluggia, Italy). Tri- glycerides were also measured with a commercially available kit (TRIG; Hoffmann-La Roche, Grenzach, Germany).
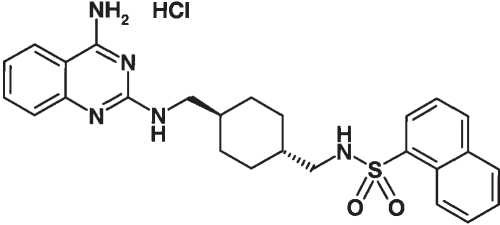
Materials. The NPY Y5 antagonist (trans-naphthalene-1-sulfonic acid {4-[(4-amino-quinazolin-2-ylamino)-methyl]-cyclohexylmethyl}- amide hydrochloride; CGP 71683A) was synthesized by Novartis Pharma AG. The structure of CGP 71683A is shown in Fig. 1. Insulin (Actrapid HM) was obtained from Novo Nordisk Pharma (Kues- nacht, Switzerland). Human NPY was obtained from Bachem (Buben- dorf, Switzerland) and FLUO-3 AM from Molecular Probes Europe BV (Leiden, Holland). The thrombin receptor agonist peptide TRAP was synthesized by Novartis Pharma AG. The cell lines stably trans- fected with the rat NPY receptors were obtained from Synaptic Phar- maceutical Corp. (Paramus, NJ).
Statistics and data analysis. Generally, where parameters were fol- lowed as a function of time, the data were statistically analyzed by univariate two-way ANOVA with repeated measures for time (see Figs. 4-5 and 7-10). The analysis incorporated the Greenhouse-Geisser correction for departures from the model. In the case of significant drug-time interactions, the Least Significant Difference (LSD) pro- cedure was used to compare the values at each time point in the drug- treated groups with the corresponding time point in the control group of animals. Two-way ANOVA followed by the LSD was used for data analysis in Fig. 3. One-way ANOVA was used for data analysis in Figs. 6 and 10 and in Table III. Unpaired t tests were used for data analysis in Table II. P < 0.05 (two-tailed) was considered to be statis- tically significant.
Figure 1. Chemical structure of CGP 71683A.
Results
Affinity of CGP 71683A to rat NPY receptor subtypes (Table I) CGP 71683A displayed high affinity for the cloned rat NPY Y5 receptor (1.460.09 nM) but bound with at least 1,000-fold lower affinity to the Y1, Y2, and Y4 NPY receptor subtypes.
Antagonism of NPY-induced intracellular calcium transients in human NPY Y5-transfected LMTK mouse fibroblasts by CGP 71683A (Fig. 2)
The antagonistic properties of CGP 71683A were assessed by measuring the ability of this compound to inhibit NPY- induced Ca2+ transients in mouse fibroblasts. In this cellular model, CGP 71683A inhibited Ca2+ transients induced by 100 nM NPY in a concentration-dependent manner (IQ0 = 5.861.2 nM, n = 4). The suppression of the maximal effects of NPY by increasing concentrations of CGP 71683A is compati- ble with an insurmountable mode of antagonism. The inhibi- tory effects of the compound appeared to be selective, since activation of thrombin receptors, which also induced Ca21 transients in this cell type, was not affected by concentrations of CGP 71683A up to 10 mM (results not shown). Further- more, compounds from our screening program that were not demonstrable NPY Y5 receptor antagonists did not affect NPY-induced Ca2+ transients in this cell line (results not shown). When tested alone, CGP 71683A did not elicit Ca21 transients up to a concentration of 10 mM, indicating that the compound is devoid of agonistic activity (results not shown).
Effect of CGP 71683A on food intake stimulated by intracerebroventricular injection of NPY (Fig. 3)
Compared with time control values, intracerebroventricular injection of 300 pmol NPY induced a prompt, significant, and sustained increase in food intake. Intraperitoneal injection of
Table I. Ability of CGP 71683A to Compete for 125I-[Pro34]hPYY Binding to Rat Y1, Y4, and Y5 Receptors or 125I-hPYY Binding to the Rat Y2 Receptor
Results are expressed as mean6SEM.
Numbers of experiments are in parentheses.
Figure 2. Antagonism of NPY-induced intracellular Ca2+ transients in LMTK mouse fibroblasts by CGP 71683A. Mouse fibroblasts ex- pressing the NPY Y5 receptor were incubated with different concen- trations of NPY. The increase in Ca2+ transients induced by NPY in these cells was antagonized dose-dependently by CGP 71683A. Re- sults are expressed as mean±SEM from five to six observations at each data point.
CGP 71683A inhibited NPY-induced feeding in a dose-depen- dent manner. The highest dose given (10 mg/kg, i.p.) signifi- cantly inhibited NPY induced food intake by z 50%, 2 h after treatment. Administration of 10 mg/kg CGP 71683A alone did not significantly affect food intake relative to control values during the 8-h period after injection (see Fig. 4).
Effect of CGP 71683A on food intake in 24-h fasted and free-feeding rats (Fig. 4)
Fasted rats. Immediately after food was returned to the con- trol animals, they ate rapidly for the first 2 h and thereafter had eaten more at each time period than observed in free- feeding rats. Injection of CGP 71683A reduced food intake in all phases of the light/dark cycle in a dose-dependent manner. The reduction in food intake induced by CGP 71683A was particularly evident during the dark phase. The highest dose of CGP 71683A administered (100 mg/kg i.p.) reduced food intake virtually completely 2 h after returning food to the animals.
Free-feeding rats. The results show that rats eat little during the light phase but steadily increase their food intake towards the beginning of the dark phase, during which the majority of their daily food intake is consumed. Injection of CGP 71683A reduced food intake in all phases of the light/dark cycle in a dose-dependent manner. The highest dose of CGP 71683A ad- ministered (100 mg/kg i.p.) resulted in essentially complete cessation of food intake for most of the experimental period.
Effect of CGP 71683A on meal pattern in free-feeding rats (Table II)
In these experiments, rats were injected with CGP 71683A (10 mg/kg i.p.) at the beginning of the light phase and changes in the
Figure 3. Effect of CGP 71683A on food intake stimulated by intra- cerebroventricular injection of NPY. Rats were injected intracere- broventricularly with either 300 pmol porcine NPY or an equivalent volume of vehicle (10 ml). Changes in food intake were measured over the subsequent 8-h period. In both cases, the 10% DMSO vehi- cle for CGP 71683A was injected intraperitoneally (2 ml/kg). Concur- rent intraperitoneal injection of 3 or 10 mg/kg CGP 71683A inhibited NPY-induced food intake in a dose-dependent manner. Results show mean±SEM from five to seven animals in each group, *P < 0.05 compared with NPY induced food intake. +P < 0.05 compared with vehicle in the group given NPY. Pattern of food intake were recorded for 24 h until the end of the dark period. The results show that total food intake as well as meal number, meal size, meal duration, and meal intervals were all significantly decreased compared with vehicle-injected con- trol animals. In contrast, the eating rate during the treatment period was not significantly affected by CGP 71683A. Time to first meal was clearly delayed by NPY Y5 receptor blockade.
Table II. Effect of CGP 71683A on the Pattern of Food Intake Measured over 24 h in Free-feeding Rats
CGP 71683A (10 mg/kg) or the 10% DMSO vehicle (2 ml/kg) was in- jected intraperitoneally at the beginning of the light phase and the above pattern of food intake was measured over 24 h while the animals were in an automated feeding system. Results are expressed as mean±SEM from 15 animals in each group. Statistics by unpaired t tests which compare the values between drug-treated and control animals for each parameter. *P < 0.05.
Figure 4. Effect of CGP 71683A on food intake in fasted and
free-feeding rats. Rats fasted for 24 h or free-feeding animals were
injected intraperitoneally either with vehicle (10% DMSO, 2 ml/kg) or CGP
71683A (1, 10, and 100 mg/kg) immedi- ately after the beginning of the
light phase (time 0). After injection of CGP 71683A, cumulative food intake
was measured at different time periods over the following 24-h period.
Results are expressed as mean±SEM
from five animals in each group of the free-feeding and seven to nine
animals in the 24-h fasted studies, *P < 0.05 vs. control.
However, because of the marked variation in this parameter, the difference was not quite statistically significant (P < 0.079).
Effect of insulin on food intake after administration of 100 mg/kg CGP 71683A to free-feeding rats (Fig. 5)
The purpose of these experiments was to investigate the ability of hypoglycemia to reverse the complete inhibition of food in- take observed after administration of 100 mg/kg CGP 71683A in free-feeding rats. In comparison to the increase in food in- take observed during the light phase in vehicle-treated free- feeding animals, food intake remained completely suppressed in animals pretreated 24 h earlier with 100 mg/kg CGP 71683A. Subcutaneous injection of insulin at the beginning of the light phase produced a similar and rapidly developing in- crease in food intake above the values recorded in either vehi- cle control or CGP 71683A-treated animals.
Effect of CGP 71683A on conditioned taste aversion (Fig. 6)
Prior treatment with lithium chloride on test day 1 induced a dose-dependent reduction in the ingestion of saccharine solu- tion on test day 2 (only the effect of the highest dose of lithium chloride is shown). When CGP 71683A was injected on test day 1, no subsequent aversion was observed at doses of 1 and 10 mg/kg, whereas a significant inhibition of the ingestion of saccharine solution was observed at a dose of 100 mg/kg. Com- parison with the changes in food intake induced by CGP 71683A in free-feeding rats (Fig. 4) indicates a clear separation between the aversive effects of the Y5 antagonist and its effects on food intake at doses of 1 and 10 mg/kg.
Effect of CGP 71683A on water intake in 24-h dehydrated/fasted rats (Fig. 7)
When injected into rats that had been both fasted and dehy- drated for 24 h, CGP 71683A did not significantly affect subse- quent water intake during the light phase. On the other hand, during the dark phase, CGP 71683A produced a progressively developing and dose-dependent inhibition of water intake, particularly towards the end of the treatment period.
Effect of CGP 71683A on food intake in streptozotocin diabetic rats (Fig. 8)
Diabetes had been successfully induced as illustrated by the low plasma insulin levels (0.76±0.13 ng/ml, n = 9) and the high.
Figure 5. Effect of insulin on food intake in rats pretreated with CGP 71683A. Rats were injected intraperitoneally either with CGP 71683A (100 mg/kg) or vehicle (10% DMSO, 2 ml/kg). 24 h later, at the begin- ning of the light phase, rats from either group were injected subcutaneously either with vehicle (0.9% NaCl, 6 ml/kg) or 60 IU insulin (time 0) and cumulative food intake was measured over the following 8-h pe- riod. Results are expressed as the mean± SEM from five to six animals in each group, *P < 0.05 vs. vehicle.
Figure
7. Effect of CGP 71683A on water
intake in dehydrated rats. Rats deprived of water for 24 h were injected
intraperitoneally either with vehicle (10% DMSO, 2 ml/kg) or CGP 71683A (1,
10, and 100 mg/kg) immediately after the beginning of the light phase (time
0). After injection of CGP 71683A, cumulative water intake was mea- sured
at different time periods over the following 24-h period. Results are
expressed as mean±SEM from six animals in
each group, *P < 0.05 vs.
control.
plasma glucose levels (620.8±68.3 ng/dl, n = 9) in the control group of animals. Injection of CGP 71683A reduced food in- take in both the dark and light periods in a dose-dependent manner. However, in comparison with their normal counter- parts, food intake during the experiment was higher in the dia- betic than in normal free-feeding rats at each dose given. This was particularly evident in animals given the highest dose of 100 mg/kg where food intake was not completely suppressed as it was in the free-feeding animals.
Chronic administration of CGP 71683A on food intake and body weight (Fig. 9)
Food intake in the control group of animals was stable and av- eraged z 27 g/d during the 28-d experimental period. Daily in- traperitoneal injection of CGP 71683A (1, 3, and 10 mg/kg per day i.p.) produced a significant and dose-dependent inhibition of food intake. The greatest fall in food intake occurred during the 3-d period after starting drug treatment. During this time period, the highest dose administered (10 mg/kg) resulted in almost complete cessation of food intake. Thereafter, despite continuous drug treatment, food intake progressively returned to control levels over the following 7 d. Body weight in these growing animals increased progressively throughout the ex- perimental period. Although CGP 71683A produced a reduc- tion in body weight at all doses administered, only the fall observed after treatment with 10 mg/kg was statistically signif- icant from controls. Despite the fact that food intake returned to control levels with time, body weight remained significantly decreased from control values for the duration of the experi- mental period.
Figure 6. Effect of CGP 71683A on conditioned taste aversion. Over a 5-d period, rats were trained to drink a solution of sodium saccha- rine (0.2%) for 20 min after a 14-h period of water deprivation. 2 d later, rats were offered saccharine solution after another 14 h of water deprivation. Thereafter, the rats were injected intraperitoneally with LiCl (10, 100, and 1,000 mmol/kg) or CGP 71683A (1, 10, and 100 mg/kg). Control animals received the 10% DMSO vehicle (2 ml/kg). 2 d later the rats were again offered the sodium saccharine solution and the quantity consumed was determined (shown in figure). Re- sults are expressed as the mean±SEM from six animals per group, *P < 0.05 vs. control.
Effect of chronic administration of CGP 71683A on food
intake, body weight, and fat pad weight (Fig. 10 and Table III)
In these experiments, rats were injected intraperitoneally with CGP 71683A (10 mg/kg per day). Daily administration of CGP
Table III. Chronic Administration of CGP 71683A on Regional Fat Depots and Plasma Triglycerides, Glucose, and Insulin
CGP 71683A was injected at the beginning of the light phase on 28 con- secutive days (10 mg/kg per day). At the end of the treatment period, the rats were killed, the fat pads were removed and weighed, and blood was taken. Results are expressed as mean±SEM from eight animals for each parameter. Statistics by one-way ANOVA. Post hoc test by the LSD procedure which compared differences between drug and pair fed groups and the control group. *Significantly different from control group at P < 0.05.
(Figure 8.)
Effect of CGP 71683A on food intake in
streptozotocin dia- betic rats. Diabetic rats were injected intraperitoneally
either with ve- hicle (10% DMSO, 2 ml/kg) or CGP 71683A (1, 10, and 100 mg/kg)
immediately after the beginning of the light phase (time 0). After in- jection
of CGP 71683A, cumulative food intake was measured at dif- ferent time periods
over the following 24-h period. Results are ex- pressed as mean±SEM from 8-10 animals in each group, *P < 0.05
vs. control.
71683A produced a significant reduction in food intake during the dark phase. A third group of animals were pair fed to those receiving CGP 71683A. Pair feeding produced a close match for food intake observed in the drug-treated animals. Chronic CGP 71683A treatment also produced the expected and main- tained fall in body weight which was paralleled by the pair fed animals. Pair feeding produced a significant fall in the weight of the perirenal and epididymal fat pads. However, despite producing identical falls in food intake and body weight to the pair fed animals, the drug-treated animals had significantly lower fat pad weights (Fig. 10). In these experiments, CGP 71683A also produced a statistically significant fall in plasma triglycerides which was identical to that observed in pair fed animals. Neither pair feeding nor the administration of CGP 71683A produced significant changes in either plasma glucose or plasma insulin concentrations.
Discussion
The purpose of these experiments was to investigate the hy- pothesis of whether NPY Y5 receptor blockade could induce acute and chronic reductions in food intake and body weight. This hypothesis was examined by studying NPY-induced feeding behavior in response to blockade of NPY Y5 receptors with a new highly selective nonpeptidic antagonist, CGP 71683A.
Within the hypothalamus, NPY is synthesized mainly in neurons of the arcuate nucleus (ARC). Axons from these cells pass through the lateral hypothalamus and release NPY onto cell bodies in the paraventricular nucleus (PVN) and the dor- somedial hypothalamus (DMH). The release of NPY in these two regions leads to the initiation of food intake. In lean rats, many studies have shown acute injections of NPY directly into the PVN and DMH or indirectly into the cerebral ventricles to substantially increase food intake in satiated rats (2, 3). Similar results are shown in the present studies.
Neurons in the ARC that synthesize and ultimately release NPY in the PVN play a key role in responding to reductions in body energy stores by initiating food intake. For example, in both fasted and streptozotocin diabetic rats, two models of en- ergy deficit, the ARC, the PVN, and the DMH all show ele- vated NPY levels (4, 6). Both animal models also exhibit in- creased NPY mRNA gene expression in the ARC while NPY release from the PVN is concurrently increased (4, 7, 9-11). In free-feeding rats, NPY mRNA within the ARC is increased on transition from the light to the dark phase, whereas NPY levels are elevated in the PVN at this time. The above observations have been interpreted to suggest that the enhanced food in- take of the dark phase as well as the hyperphagia of energy- deprived rats are due to increased central NPY levels.
A temporal relationship between increased
hypothalamic NPY levels and food intake has been established (12). Further-
more, inhibition of NPY synthesis or blockade of the action of
Figure 9. Effect of chronic administration of CGP 71683A on food in- take and body weight. Rats were injected intra- peritoneally with differ- ing doses of CGP 71683A or an equal volume of the 10% DMSO vehicle (2 ml/kg). Injections were given daily at the begin- ning of the light phase for a total of 28 d. Results are expressed as mean± SEM from six animals in each group, *P < 0.05 vs. control.
Figure 10. Effect of chronic administration of CGP 71683A on food
intake, body weight, and fat pad weight. After a 3-d control period, rats
were injected intraperitoneally with CGP 71683A (10 mg/kg) or the 10% DMSO
vehicle (2 ml/kg). Injections were given daily at the beginning of the
light phase for a total of 28 d. A separate group of rats was pair fed
every 12 h to the changes in food intake occurring in the CGP 71683A-
treated animals 24 h before. The symbols representing the pair fed animals
are obscured since they lie almost exactly over those representing food
intake in the drug-treated animals. At the end of the treatment period, the
rats were killed and the fat pads were removed and weighed. Re- sults are
expressed as mean±SEM. There are eight
rats in each group. *P < 0.05 vs. control. aP < 0.05 vs. control. abP < 0.05, significantly differ- ent from control and pair
fed group.
NPY with antibodies or peptide receptor antagonists has been shown to suppress food intake in free-feeding and food- deprived rats (14-16). These studies, along with the changes in NPY levels in hypothalamic nuclei described above, all suggest that NPY plays a physiologically important role in the control of food intake in both normal and energy-deprived rats.
In binding studies using cell lines stably expressing rat NPY receptors, CGP 71683A was shown to have a high affinity for the rat NPY Y5 receptor subtype (ICs0: 1.4 nM). In contrast, CGP 71683A bound to rat Y1, Y2, and Y4 receptors with at least 1,000-fold lower affinity. Among other rat receptors tested, the lowest selectivity was found against the rat a-adren- ergic 2c receptor subtype (63-fold). Measurement of intracel- lular Ca2+ transients in LMTK cells stably expressing the hu- man NPY Y5 receptor indicated that CGP 71683A was devoid of agonistic activity and blocked NPY-induced Ca2+ transients through an insurmountable antagonism. Therefore, CGP 71683A can be considered as a potent and selective antagonist of the rat NPY Y5 receptor.
The increase in food intake induced by intracerebroventric- ular injection of exogenous NPY could be significantly inhib- ited by prior administration of CGP 71683A, suggesting that this compound enters the brain and is an effective antagonist of NPY-induced feeding acting through the NPY Y5 receptor.
In these studies, the NPY receptor antagonist CGP 71683A decreased food intake in streptozotocin diabetic, food-deprived, and free-feeding rats. In free-feeding rats, CGP 71683A dose- dependently decreased food intake during the dark phase, a time when NPY levels in the hypothalamus are markedly in- creased. At a dose of 10 mg/kg, CGP 71683A reduced food intake by z 50%. The maintenance of a normal pattern of feed- ing behavior and the absence of demonstrable aversion would indicate that the observed reduction in food intake was not due to a nonspecific effect of the compound. Indeed, experi- ence with this compound has shown it to be well tolerated at low dosages. At the highest dose studied (100 mg/kg), food in- take was completely suppressed in free-feeding and fasted rats. The failure to eat after administration of the compound at 100 mg/kg was probably because of the marked aversion that was induced and which was reflected in observations of ruffled fur and a tendency towards reduced motility at this dosage. How- ever, the animals were able to respond to the injection of a hypoglycemic dose of insulin with a robust increase in food in- take. Therefore, at least one food stimulatory pathway re- mained intact and the animals were able to eat (but chose not to), after administration of CGP 71683A. Interestingly, dried brown nasal secretions were observed in animals 24 h after treatment with 100 mg/kg CGP 71683A and in a lighter and less uniform way in animals treated with 10 mg/kg CGP 71683A. The signif- icance of these findings is not known but appeared not to be visually related either to the health of the animals or to their reduced food intake at low doses. However, a possible expla- nation may involve the known role of NPY in the control of nasal secretion (32). Interestingly, food intake in streptozoto- cin diabetic rats was not completely suppressed by the highest dose of CGP 71683A, suggesting that factors other than NPY contribute to the hyperphagia of this animal model. Clearly, the relative role of NPY and other neurotransmitters in the con- trol of food intake in diabetic rats remains to be determined.
Not only did the administration of 10 mg/kg CGP 71683A acutely lower food intake in free-feeding rats it also did so when administered chronically. However, after 3-4 d of in- creasing efficacy, food intake returned progressively back to- wards normal and remained there for the duration of the 28-d treatment period. This result would indicate that with contin- ued NPY blockade counterregulatory mechanisms are induced to prevent starvation. This is not a surprising finding given the many overlapping systems that operate to control food intake (33). The return of food intake to control levels with chronic treatment is also seen with other appetite suppressants such as dexfenfluramine (34). Interestingly, in the present studies body weight fell with chronic NPY Y5 receptor blockade and remained suppressed even though food intake had returned to normal. This fall in body weight clearly occurred in two phases. The first paralleled the fall in food intake whereas the second did not and was associated at the end of the experiment with a decrease in fat pad weight below that observed in pair fed ani- mals. It is possible that the additional decrease in fat pad weight may have been due to nonspecific effects related to the proximity of the very high local concentrations of CGP 71683A present at the injection site. However, in the brain, NPY has two effects on energy metabolism in addition to in- crease feeding. These are decreased brown fat thermogenesis and increased white fat lipoprotein lipase activity, both of which are secondary to increased central sympathetic outflow. The later fall in body weight and decrease in fat pad weight af- ter chronic administration of CGP 71683A may then have been due to an increase in central sympathetic outflow rather than a decrease in food intake (35).
After release within the appetite-regulating centers of the brain, NPY acts on specific postsynaptic receptors to initiate food intake. NPY receptors, including the NPY Y5 subtype, are present in the hypothalamus. However, despite extensive investigation, the subtype mediating the effects of NPY on food intake has remained controversial. Preliminary evidence suggesting a role for NPY Y5 receptors in the control of feed- ing came from the observation that the ability of a wide range of NPY peptide analogues to stimulate food intake correlated best with their affinity for the NPY Y5 subtype (18, 19). These observations were supplemented recently by experiments demonstrating that intracerebroventricular injection of anti- sense oligonucleotides directed against the NPY Y5 receptor inhibited both NPY and fasting induced food intake (20, 21). That the NPY Y5 receptor is intimately involved in the re- sponse of food intake to NPY is given strong support by the re- sults of the present study demonstrating that a highly selective NPY Y5 antagonist inhibited NPY-induced food intake as well as appetite in free-feeding and energy-deprived rats.
Although accumulating evidence strongly supports a role for the NPY Y5 receptor in NPY-induced food intake, there is conflicting evidence for the involvement of the NPY Y1 receptor subtype. For example, although peptide and nonpeptide NPY Y1 antagonists have been shown to inhibit both NPY and fasting-induced food intake, the known pharmacology of this receptor is not consistent with pharmacology of known NPY peptides which stimulate feeding. Furthermore, central admin- istration of antisense oligonucleotides directed against the NPY Y1 receptor or injection of a selective Y1 antagonist appear to increase or decrease food intake depending on the study (2326, 36). These paradoxical observations could be interpreted to suggest that the NPY Y1 receptor has dual activities in the con- trol of NPY-induced food intake. Interestingly, inhibition of NPY Y1 receptors has been shown to induce anxiety which may be a confounding factor contributing to the changes in food intake observed after blockade of NPY Y1 receptors. Al- though evidence exists to support the involvement of NPY Y5 and perhaps NPY Y1 receptors in the control of food intake, other studies with selective peptide agonists suggest that other as yet unidentified receptor subtypes may also be involved (22, 23). Clearly, additional studies are needed to further define the relative contribution of receptor subtypes in addition to NPY Y5 in the control of NPY-induced food intake.
The role of NPY in the control of appetite is now well es- tablished, but its involvement in the control of drinking, an as- sociated behavior, is less well characterized. Central adminis- tration of NPY stimulates water intake in rodents and rabbits, an effect believed to be independent of its orexigenic effects (37, 38). Recent studies have demonstrated increased prepro- NPY mRNA levels in the ARC after water deprivation (39). These observations suggest that central NPY independently stimulates water as well as food intake. In these studies, CGP 71683A significantly inhibited dehydration-induced drinking in the absence of changes in food intake, suggesting that en- dogenous NPY acting through NPY Y5 receptors is indepen- dently involved in the control of both food and water intake.
In summary, the highly selective NPY Y5 antagonist CGP 71683A antagonized NPY-induced feeding and inhibited spon- taneous food intake in diabetic, 24-h fasted, and free-feeding rats. With prolonged NPY Y5 receptor blockade, the initial dramatic fall in food intake in free-feeding rats became pro- gressively attenuated over time, suggesting that counterregula- tory systems are induced to return food intake to normal. Al- though food intake returned to normal with prolonged NPY Y5 receptor blockade, body weight remained low. These data strongly support the hypothesis that the NPY Y5 receptor sub- type is involved in NPY-induced food intake and further sug- gest that NPY Y5 antagonists have a role to play in the treat- ment of obesity.
Acknowledgments
We would like to thank S. di Bello, D. Eichlisberger, D. Folio, L. Hartmann, F. Kilcher, F. Lugrin, D. Mannhart, G. Monnat, O. Peter, K. Pfeiffer, R. Schwaller, Z. Shaposhnik, H. Thomann, R. Wicki, and S. Zhou for technical assistance.
References
- Heilig, M., and E. Widerlov. 1995. Neurobiology and clinical aspects of neuropeptide Y. Crit. Rev. Neurobiol. 9:115-136.
- Stanley, B.G., and S.F. Leibowitz. 1984. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 35: 2635-2642.
- Levine, A.S., and J.E. Morley. 1984. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 5:1025-1029.
- Sahu, A., C.A. Sninsky, P.S. Kalra, and S.P. Kalra. 1990. Neuropeptide-Y concentration in microdissected hypothalamic regions and in vitro release from the medial basal hypothalamus-preoptic area of streptozotocin-diabetic rats with and without insulin substitution therapy. Endocrinology. 126:192-198.
- Sahu, A., P.S. Kalra, and S.P. Kalra. 1988. Food deprivation and inges- tion induce reciprocal changes in neuropeptide Y concentrations in the paraventricular nucleus. Peptides. 9:83-86.
- Williams, G., J.S. Gill, Y.C. Lee, H.M. Cardoso, B.E. Okpere, and S.R. Bloom. 1989. Increased neuropeptide Y concentrations in specific hypotha- lamic regions of streptozocin-induced diabetic rats. Diabetes. 38:321-327.
- Schwartz, M.W., A.J. Sipols, C.E. Grubin, and D.G. Baskin. 1993. Differ- ential effect of fasting on hypothalamic expression of genes encoding neuropep- tide Y, galanin, and glutamic acid decarboxylase. Brain Res. Bull. 31:361-367.
- Beck, B., A. Stricker Krongrad, A. Burlet, J.P. Nicolas, and C. Burlet. 1990. Influence of diet composition on food intake and hypothalamic neu- ropeptide Y (NPY) in the rat. Neuropeptides. 17:197-203.
- Hanson, E.S., N. Levin, and M.F. Dallman. 1997. Elevated corticosterone is not required for the rapid induction of neuropeptide Y gene expression by an overnight fast. Endocrinology. 138:1041-1047.
- Marks, J.L., K. Waite, and M. Li. 1993. Effects of streptozotocin- induced diabetes mellitus and insulin treatment on neuropeptide Y mRNA in the rat hypothalamus. Diabetologia. 36:497-502.
- Brady, L.S., M.A. Smith, P.W. Gold, and M. Herkenham. 1990. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food- deprived rats. Neuroendocrinology. 52:441-447.
- Sahu, A., C.A. Sninsky, and S.P. Kalra. 1997. Evidence that hypotha- lamic neuropeptide Y gene expression and NPY levels in the paraventricular nucleus increase before the onset of hyperphagia in experimental diabetes. Brain Res. 755:339-342.
- Jhanwar Uniyal, M., B. Beck, C. Burlet, and S.F. Leibowitz. 1990. Diur- nal rhythm of neuropeptide Y-like immunoreactivity in the suprachiasmatic, ar- cuate and paraventricular nuclei and other hypothalamic sites. Brain Res. 536: 331-334.
- Hulsey, M.G., C.M. Pless, B.D. White, and R.J. Martin. 1995. ICV ad- ministration of anti-NPY antisense oligonucleotide: effects on feeding behav- ior, body weight, peptide content and peptide release. Regul. Pept. 59:207-214.
- Shibasaki, T., T. Oda, T. Imaki, N. Ling, and H. Demura. 1993. Injection of anti-neuropeptide Y gamma-globulin into the hypothalamic paraventricular nucleus decreases food intake in rats. Brain Res. 601:313-316.
- Dube, M.G., B. Xu, W.R. Crowley, P.S. Kalra, and S.P. Kalra. 1994. Ev- idence that neuropeptide Y is a physiological signal for normal food intake. Brain Res. 646:341-344.
- Blomqvist, A.G., and H. Herzog. 1997. Y-receptor subtypes: how many more? Trends. Neurosci. 20:294-298.
- Gerald, C., M.W. Walker, L. Criscione, E.L. Gustafson, C. Batzl Hart- mann, K.E. Smith, P. Vaysse, M.M. Durkin, T.M. Laz, D.L. Linemeyer, et al. 1996. A receptor subtype involved in neuropeptide-Y-induced food intake. Na- ture. 382:168-171.
- Wyss, P., A. Stricker Krongrad, L. Brunner, J. Miller, A. Crossthwaite, S. Whitebread, and L. Criscione. 1998. The pharmacology of neuropeptide Y (NPY) receptor-mediated feeding in rats characterizes better Y5 than Y1, but not y2 or Y4 subtypes. Regul. Pept. 75:363-371.
- Schaffhauser, A.O., A. Stricker Krongrad, L. Brunner, F. Cumin, C. Gerald, S. Whitebread, L. Criscione, and K.G. Hofbauer. 1997. Inhibition of food intake by neuropeptide Y Y5 receptor antisense oligodeoxynucleotides. Diabetes. 46:1792-1798.
- Tang Christensen, M., P. Kristensen, and P.J. Larsen. 1997. Central ad- ministration of NPY Y5 receptor antisense oligonucleotides reduces food in- take and blocks NPY induced feeding in male rats. Society Neurosci. 23:1345. (Abstr.)
- Broqua, P., J.G. Wettstein, M.N. Rocher, B. Gauthier Martin, P.J. Riviere, J.L. Junien, and S.G. Dahl. 1996. Antinociceptive effects of neuropep- tide Y and related peptides in mice. Brain Res. 724:25-32.
- O'Shea, D., D.G. Morgan, K. Meeran, C.M. Edwards, M.D. Turton, S.J. Choi, M.M. Heath, I. Gunn, G.M. Taylor, J.K. Howard, et al. 1997. Neuropep- tide Y induced feeding in the rat is mediated by a novel receptor. Endocrinol- ogy. 138:196-202.
- Lopez Valpuesta, F.J., J.W. Nyce, T.A. Griffin Biggs, J.C. Ice, and R.D. Myers. 1996. Antisense to NPY-Y1 demonstrates that Y1 receptors in the hypo- thalamus underlie NPY hypothermia and feeding in rats. Proc. R. Soc. Lond. B. Biol. Sci. 263:881-886.
- Heilig, M. 1995. Antisense inhibition of neuropeptide Y (NPY)-Y1 receptor expression blocks the anxiolytic-like action of NPY in amygdala and par- adoxically increases feeding. Regul. Pept. 59:201-205.
- Kanatani, A., A. Ishihara, S. Asahi, T. Tanaka, S. Ozaki, and M. Ihara. 1996. Potent neuropeptide Y Y1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology. 137: 3177-3182.
- Doods, H.N., H.A. Wieland, W. Engel, W. Eberlein, K.D. Willim, M. Entzeroth, W. Wienen, and K. Rudolf. 1996. BIBP 3226, the first selective neu- ropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul. Pept. 65:71-77.
- Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bind- ing. Anal. Biochem. 72:248-254.
- Stricker Krongrad, A., B. Beck, and C. Burlet. 1996. Enhanced feeding response to neuropeptide Y in hypothalamic neuropeptide Y-depleted rats. Eur. J. Pharmacol. 295:27-34.
- Larue, C. 1978. Oral cues involved in the rat's selective intake of fats. Chem. Senses Flavour. 3:1-6.
- Ramirez, I. 1992. Chemoreception for fat: do rats sense triglycerides di- rectly? Appetite. 18:193-206.
- Lacroix, J.S., and B.L. Mosimann. 1996. Attenuation of allergen-evoked nasal responses by local pretreatment with exogenous neuropeptide Y in atopic patients. J. Allergy Clin. Immunol. 98:611-616.
- Levine, A.S., and C.J. Billington. 1997. Why do we eat? A neural sys- tems approach. Annu. Rev. Nutr. 17:597-619.
- Li, B.H., and N.E. Rowland. 1996. Effect of chronic dexfenfluramine on Fos in rat brain. Brain Res. 728:188-192.
- Billington, C.J., J.E. Briggs, S. Harker, M. Grace, and A.S. Levine. 1994. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am. J. Physiol. 266:R1765-R1770.
- Schaffhauser, A.O., S. Whitebread, R. Haener, K.G. Hofbauer, and A. Stricker Krongrad. 1998. Neuropeptide Y Y1 receptor antisense oligodeoxynu- cleotides enhance food intake in energy-deprived rats. Regul. Pept. 75:417-423.
- Morley, J.E., and J.F. Flood. 1989. The effect of neuropeptide Y on drinking in mice. Brain Res. 494:129-137.
- Pau, M.Y., K.Y. Pau, and H.G. Spies. 1988. Characterization of central actions of neuropeptide Y on food and water intake in rabbits. Physiol. Behav. 44:797-802.
- O'Shea, R.D., and A.L. Gundlach. 1995. NPY mRNA and peptide im- munoreactivity in the arcuate nucleus are increased by osmotic stimuli: correla- tion with dehydration anorexia. Peptides. 16:1117-1125.
Reduction of Food Intake by a Neuropeptide Y Y5 Antagonist CGP 71683A